Application to rheumatic heart disease and ventricular remodelling
Collaborators: Prof Sebastian Skatulla (University of Cape Town), Prof Ntobeko Ntusi (University of Cape Town), Prof Freedom Gumedze (University of Cape Town, Prof Richard Naidoo (University of Cape Town) and Dr Jagir Hussan (University of Auckland)
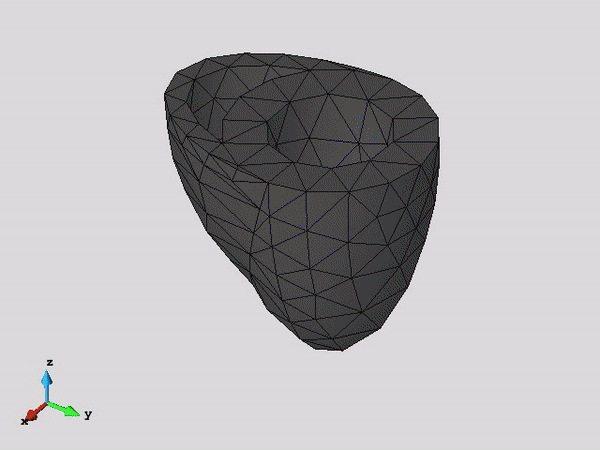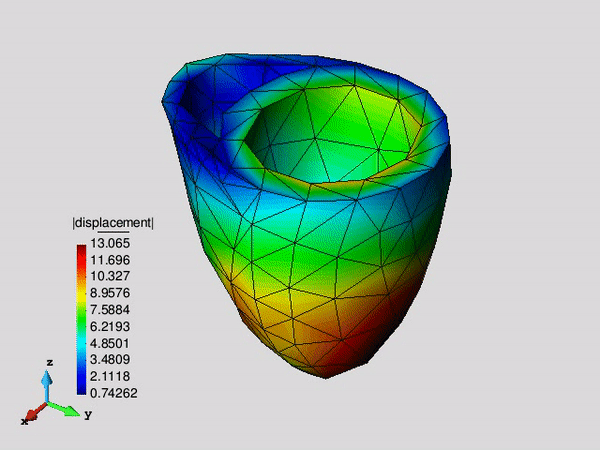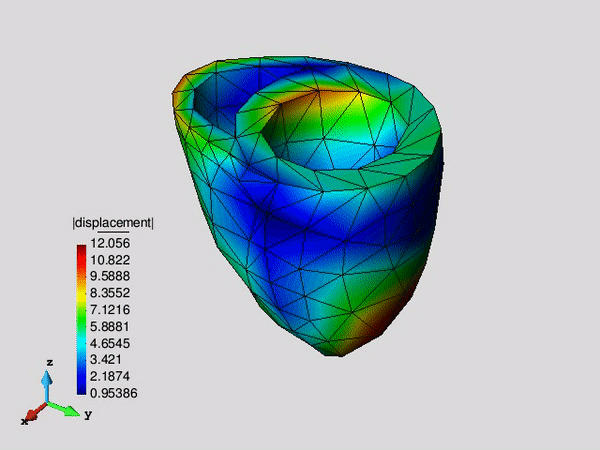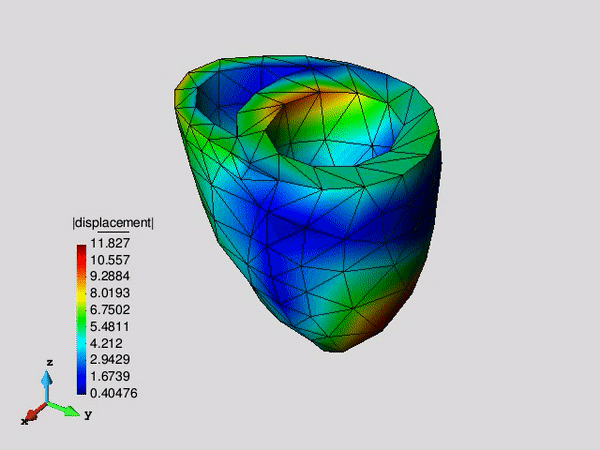
Figure 1. Systolic fibre stress distribution in an idealized left ventricle in three different pathological states: healthy, infarcted and infarcted with injection treatment .
Summary
Heart failure accounts for one in eight deaths in USA and almost 10% of individuals over the age of 65 are affected by heart failure. In particular acute rheumatic fever (ARF) and rheumatic heart disease (RHD) is a major cause of mortality amongst adolescents in third world and developing countries with 233,000 deaths annually. The failure mechanisms of RHD are not well studied in comparison to myocardial infarction which prevents effective treatment of the diseased myocardium.
Computational cardiac mechanics is emerging as a rapidly expanding area of research bringing together multidisciplinary research centred on understanding the electrophysiological and mechanical behaviour of the heart at scales ranging from cell to tissue and organ levels. Principles of continuum mechanics are key in creating a realistic multi-scale model of the heart. They allow to describe the directly observable behaviour of the heart by incorporating on micro level its complex heterogeneous and anisotropic structure as well as the coupling mechanisms between mechanical fields on the one hand and chemical and electrical fields on the other. Computational models therefore help to quantify the mechanical environment in health, injury, disease, as well as to identify mechanosensitive responses and their mechanisms. This leads to advance in therapeutic and diagnostic procedures.
In contrast to existing computational models, we want to feed our cardiac mechanics models with realistic patient-specific material properties from magnetic resonance imaging (MRI) or 3D echocardiography. This high accuracy with regards to material properties can only be exploited, if the geometric model of the heart features a similar degree of accuracy. The increase in geometry and material detail, however, is matched by an exponential increase in computing time such that it inevitably results in massive supercomputer simulations.
The novelty of our approach is to pair unparalleled accuracy with fast computing time such that it is usable on a normal computer and provides instant real-time feedback. This will be achieved by a special model reduction technique, the Proper Orthogonal Decomposition with Interpolation method (PODI). The approach will open a new dimension for heart diagnostics and provide medical researchers as well as practitioner with a computational toolbox enabling them to gain in-depth understanding of the mechanical, electro-physiological and molecular processes and coupled mechanisms which helps in developing new therapy concepts for rheumatic heart disease.
Objectives
-
development of a novel computational cardiac mechanics toolbox facilitating real-time and patient-specific simulation of the heart's function
-
development of a novel full-field characterisation of myocardial material properties based on 3D echocardiography
-
development of a novel micro-structurally motivated framework for active behaviour and growth of myocardial tissue
-
improve the lacking understanding of the chronology of RHD and the stress induced proliferative mechanisms due to chronic volume overload from the biomechanics perspective
-
study and predict the physiological changes at cellular, tissue and organ level, and the impact of current therapies on the pumping function of rheumatic hearts
-
advancement of RHD therapies and prevention of heart failure
Figure 2. Left Ventricular Fibre Stress a) Healthy Case during Diastole b) Healthy Case during IVC c) Infarcted Case during IVC
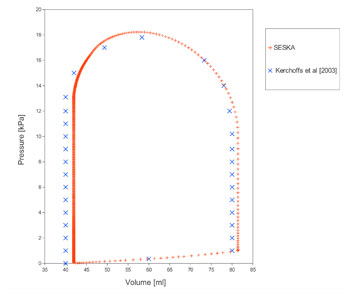
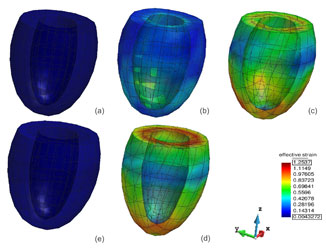
Figure 3. Left Ventricular Fibre pressure-volume curve and corresponding strain contour plots during one heart beat
